Multifocal motor neuropathy (MMN) is a progressive, scarce disorder with an estimated prevalence of < 1:100 0001, 2. Acquired immune-mediated mononeuropathy exclusively involves motor nerves; conduction block is typical and can be detected on electrophysiological examination. Weakness is focal and asymmetric, typically following the distribution of at least two motor nerves. It usually starts and preponderates in the upper extremities with no sensory involvement. The most commonly used criteria to set up the diagnosis were developed by the European Federation of Neurological Societies and Peripheral Nerve Society3, including further supportive and exclusion criteria besides those mentioned above clinical and neuro-physiological characteristics.
Nerve dysfunction in MMN has recently been linked to an antibody-mediated attack of the node of Ranvier and the paranodal region, resulting in complement activation. Ganglioside GM1 is most abundant in the nodal and paranodal regions of the peripheral motor rather than sensory nerves4. Significant titers of serum GM1 IgM antibodies are present in about half of the patients; GM1/galactocerebroside (GM1/GalC) complexes can be detected in up to 70% of the cases5, 6, aiding the identification of MMN patients. Occasionally MMN patients present with anti-GM2 and anti-GD1b IgM antibodies. Intravenous immunoglobulin (IVIG) remains the gold standard in the therapy of MMN patients; responsiveness to this therapy can be helpful to support the diagnosis in patients with a clinical phenotype compatible with MMN that do not show conduction block on electrophysiological examination.
As the pathophysiology of MMN and the exact mechanism of action of the IVIG therapy are not fully understood7, 8, we searched for additional biomarkers. Oxidative stress was proven to contribute to numerous autoimmune disorders9 and peripheral neuropathies10, leading, among others, to axonal degeneration11. ADMA is known to inhibit nitric oxide (NO) production in a concentration-dependent manner12, which blocks NO’s inhibitory effect on monocyte and leukocyte adhesion and superoxide radical release. Although both ADMA and SDMA levels have been reported to increase in the state of chronic inflammation13, no data exist for MMN. Hence, our aim in the present prospective study was to determine L-arginine, ADMA, and SDMA levels of MMN patients and to investigate the effect of IVIg therapy on the L-arginine/NO pathway.
Materials and methods
PARTICIPANTS
Ten previously diagnosed and IVIG-responsive MMN patients were enrolled in the study between 2016 and 2020. All patients (seven males and three females, mean age 52.8 ± 12.2 years, age range 38-72 years) fulfilled the Criteria of the European Federation of Neurological Societies/Peripheral Nerve Society Guideline for definite multifocal motor neuropathy3. Disease duration varied between three and 16 years (mean 8 ± 4.4 years), and the elapsed time between disease onset and the diagnosis of MMN was one-six years.
Table 1. Clinical data of patients with multifocal motor neuropathy (MMN)
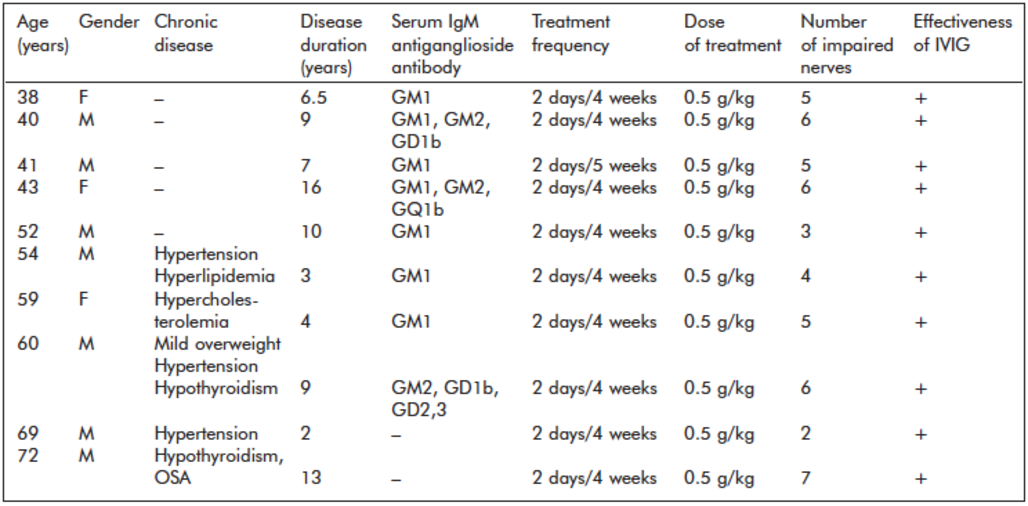
At the onset of MMN, all patients were free of any medical comorbidity, and all later developed comorbidities were treated in the study period. List of abbreviations: F: female, M: male, IVIG: intravenous immunoglobulin, OSA: obstructive sleep apnea
Healthy controls were recruited from the staff of the city university through bulletin board advertisements. Ten age- and sex-matched controls were selected from the 50 screened individuals (seven males and three females, mean age 51.6 ± 11.2 years, age range 38-69 years).
All participants were carefully tested for chronic medical conditions, which could modify serum dimethylarginine levels. Healthy controls were free of any medical conditions. MMN patients had no comorbidities at the onset of the disease and the diagnosis, but five patients developed medical conditions that had to be treated before and during the study period. Clinical data of patients are shown in Table 1. The study was approved by the Regional Research Ethics Committee of the Medical Center University (#47414) in 2017. Patients and controls signed written informed consent.
EMG/ENG STUDIES
The electrophysiological measurements were performed using the Medtronic Keypoint G4-3 channel EMG/NCV unit (Alpine Biomed APS, Tonsbakken 16-18., DK-2740 Skovlunde, Denmark). The recordings of nerve conduction studies (NCS) of peripheral nerves were performed with surface electrode patches attached to the skin, while the electromyography (EMG) studies of striated muscles weredone by disposable concentric needles (Natus Neu- rodiagnostic Supplies).
In diagnosing MMN patients, other electrophysiological investigations were performed beyond the standard MMN protocol to rule out the presence of any type of motor neuropathy disease that can mimic MMN. During the follow-up period, each patient had EMG/NCV study at least once a year routinely or more times in case of clinical worsening.
In the study protocol, each patient underwent NCV studies twice, on the first day before IVIG treatment and on the second day after IVIG therapy. The bilaterally investigated mixed-type nerves were the following: median nerve, ulnar nerve, radial nerve, deep branch of the peroneal nerve and tibial nerve. The temperatures of the limbs were between 32 and 36 °C. On both days, all the electric stimulations were performed at the same place with the same electric current intensity (mA)/same motor nerve. ENG (electroneurography) criteria for motor nerve improvement were as follow: the before and after treatment amplitude differences of individual nerves were automatically calculated by the software program of the EMG/NCV unit in percentage; if the post-treatment values of conduction blocks (CBs) showed decreased amplitude differences of at least 20% or more, we evaluated it as a positive response to the treatment.
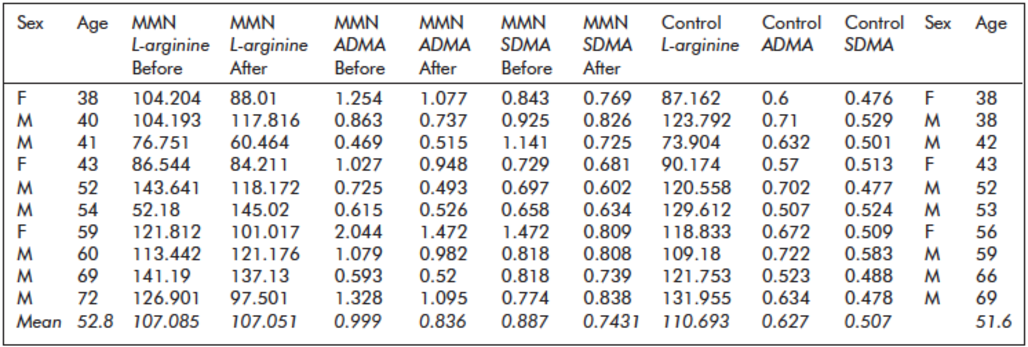
Table 2. Fasting serum L-arginine, asymmetric and symmetric dimethylarginine (ADMA, SDMA) levels (µmol/L) of patients with multifocal motor neuropathy (MMN) before and after intravenous immunoglobulin treatment and of healthy controls
IVIG TREATMENT
In the follow-up period, the dosage and frequency of IVIG treatments fit the clinical symptoms. In the study period, all patients received IVIG therapy (Human Intravenous Immunoglobulin, 10% liquid, manufactured by CSL Behring AG and distributed by CSL Behring LLC, Germany) with a frequency of 2 consecutive days/4 weeks in a 0.5 g/kg daily dose that proved to be effective without adverse effects. Regarding fine hand movements and limb strength, MMN patients were asked to evaluate the effectiveness of IVIG treatment.
STATISTICAL ANALYSES
Statistical analyses were performed using IBM SPSS Software 20.0 (IBM Corp., Armonk, NY). First, the distribution of all study variables (for controls and patients before and after IVIG therapy) was investigated by applying the Kolmogorov- Smirnov Test.
Univariate analyses: differences in continuous variables (age, L-arginine, ADMA, SMDA) bet- ween patients and controls were compared using Independent-Samples T-Test, and changes in lab variables in patients after versus before IVIG therapy (L-arginine, ADMA, SMDA) were assessed with Paired-Samples T-Test.
Multivariate analyses: we tested predictors of L- arginine, ADMA, and SMDA levels by applying stepwise multiple linear regression analyses. Age was included as an independent variable along with the grouping variable (patient vs control or patients before and after treatment) and L-arginine, ADMA, and SMDA as the dependent variable. The assumptions of multiple linear regression were satisfied as judged by testing for linearity and normality and after excluding outliers (ADMA 1 Patient, SDMA 2 Patients).
Finally, we carried out Pearson’s correlation to control for internal dependencies among the three laboratory parameters (L-arginine, ADMA, SMDA) in patients and controls; and to analyze possible correlations in patients among the lab men- tioned above variables and the presence of antiganglioside antibodies, the number of impaired motor nerves and disease duration. The level of statistical significance was set as p < 0.05.
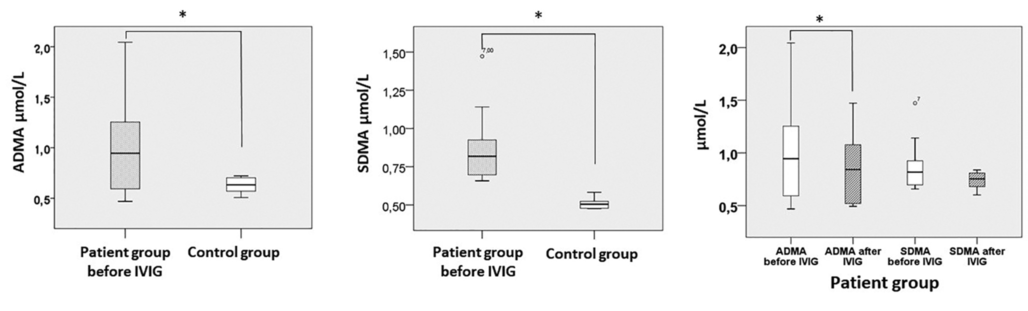
Figure 1. Asymmetric and symmetric dimethylarginine (ADMA and SDMA) serum concentrations in MMN patients and controls, as well as before and after IVIG treatment in MMN patients. Whiskers are set at minimum and maximum, the horizontal line represents the median, and the box depicts the interquartile range (25-75%). Outliers are marked with a circle. Significant differences between groups are indicated by a * on the plots
Results
ADMA, SDMA, AND L-ARGININE LEVELS IN MMN PATIENTS
Before treatment, serum concentrations of ADMA proved to be significantly higher (p = 0.0048) in MMN patients (0.99 ± 0.48) than in healthy controls (0.63 ± 0.08) (Table 2, Figure 1). Similarly, we found significantly elevated serum concentrations of SDMA before treatment (p = 0.001) in patients (0.88 ± 0.25) versus controls (0.51 ± 0.03) (Table 2, Figure 1). Controlling for the covariant age with stepwise multiple linear regression, no change in the significance pattern could be observed; ADMA (B = -0.474; p = 0.041) or SDMA (B = -0.896; p < 0.0005) serum levels remained significant predictors of the presence of MMN. We could not find a significant difference in L-arginine levels between patients (107.1 ± 28.9) and controls (110.7 ± 200).
EFFECT OF IVIG THERAPY ON SERUM LEVELS OF ADMA, SDMA, AND L-ARGININE
After IVIG therapy ADMA levels were significantly reduced in MMN patients (p = 0.025; 0.99 ± 0.48 and 0.84 ± 0.33, respectively) (Table 2, Figure 1); SDMA levels also showed a trend towards decrease (p = 0.1; 0,88 ± 0.25 and 0.74 ± 0.08 respectively); however, it did not reach significance (Table 2, Figure 1).
Furthermore, ADMA levels were positively correlated with SDMA levels in all participants as before treatment (R = 0.659; p = 0.002) as well as in patients after treatment (R = 0.636; p = 0.048). No significant correlation was found among all lab variants and age before treatment and among age, L-arginine and dimethylarginine after treatment.
IVIG therapy does not seem to influence serum L-arginine concentrations significantly (after IVIG 107.1 ± 25.8).
Being a progressive disorder, as expected, the number of affected nerves correlated with the disease duration of MMN (R = 0.679; p = 0.031). Nevertheless, we found that L-arginine, ADMA, and SDMA levels did not correlate with the number of affected nerves, disease duration, or the presence of ganglioside antibodies.
ENG FINDINGS
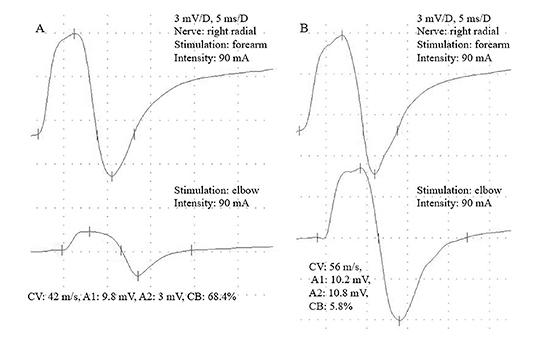
All patients reported better motor function after IVIG treatment. Altogether 49 peripheral motor nerves were found with conduction block (CB), typical for MMN pathology. Out of them, 37 (75,5%) nerves responded well to IVIG treatment (≥20% improvement of the amplitude increment). There was no patient with worsening (Table 1, Figure 2).
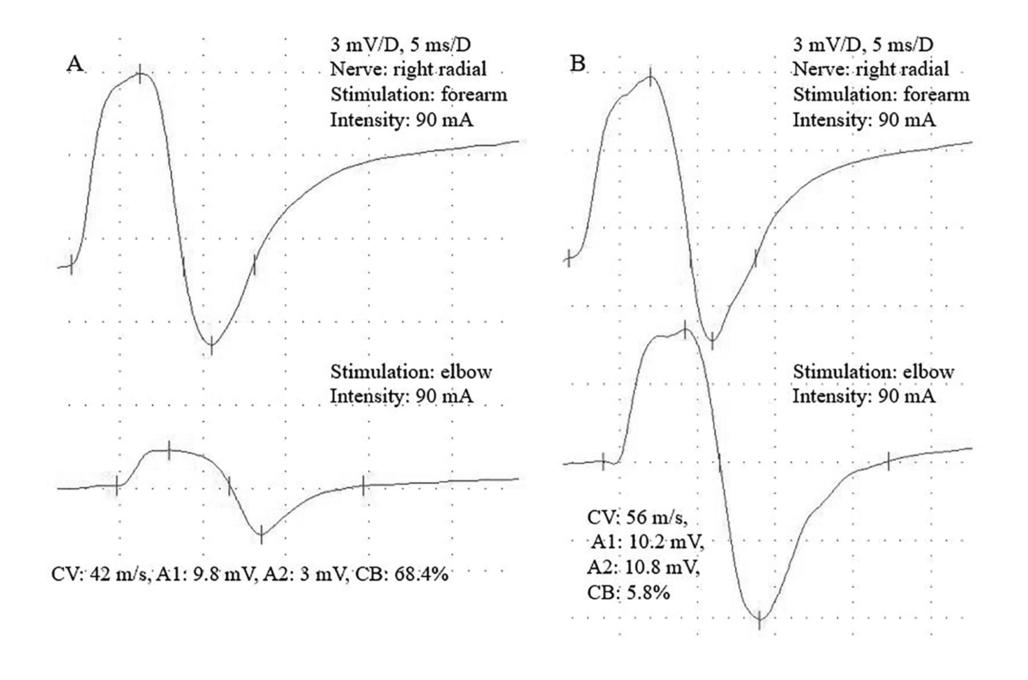
Figure 2. A 69-year-old MMN patient with nerve conduction velocity (NCV) study. Figure 2A shows severe conduction block (CB) before IVIG treatment, while the posttreatment measurements demonstrate complete recovery (2B)
DISCUSSION
We demonstrated elevated serum concentrations of ADMA and SDMA along with normal L-arginine concentrations in MMN patients and reduced ADMA and SDMA after IVIG therapy. Nitric oxide/nitrogen monoxide (NO) is produced by endothelial cells and plays a crucial role in neuroprotection. L- arginine is a natural amino acid and is NO’s primary precursor. Regulation of NO production is not limited by circulating L-arginine but by intracellular substrate and co-factor availability, NOS activation by phosphorylation, and inhibition of NOS by the endogenously methylated L-arginine derivate ADMA14. Dimethylarginines naturally occur in human plasma and are formed during the proteolysis of methylated proteins. ADMA is a known mediator of oxidative stress via the concentration-dependent suppression and uncoupling of nitric oxide synthase (NOS)15, meaning that the oxidation of L- arginine to NO is incomplete, molecular oxygen acts as an electron acceptor, leading to superoxide radical (SOR) production. This process increases the expression of genes mediating inflammatory response16. In turn, inflammation promotes the activity of protein arginine methyltransferases and impedes that of dimethylarginine dimethylamino-hydrolases (DDAHs)17, which are responsible for the metabolism of ADMA, leading to elevated dimethylarginines18 and reinforcing the vicious circle.
SDMA, the structural isomer of ADMA, has no direct inhibitory effect on NO production but blocks competitively cellular L-arginine uptake, indirectly influencing NO’s bioavailability by diminishing substrate availability19. SDMA also triggers inflammation by enhancing the production of reactive oxygen species (ROS) in vitro by modulation of stored-operated calcium channels20. When an overload of free radicals (reactive oxygen species – ROS) cannot gradually be destroyed, their accumulation in the body generates a phenomenon called oxidative stress. Under oxidative stress conditions, ROS production is dramatically increased, resulting in subsequent alteration of membrane lipids, proteins, and nucleic acids21. Excessive production of ROS leads to the oxidative post-translational modifications of proteins. These may result in neoepitopes pinpointed by the immune system as antigens and persuade the formation of autoantibodies9 (Figure 3).
B cells and autoantibodies play a vital role in the pathogenesis of autoimmune peripheral nerve diseases. Autoantibodies against peripheral nerve molecules such as gangliosides, proteins of the Ranvier node, or myelin-associated glycoprotein have been described, allowing the identification of subgroups of patients with specific clinical phenotypes. The T cell activation process includes co-stimulatory molecules and key cytokines and discusses the role of antibodies against peripheral nerve glycolipids or glycoproteins. Special attention is given to the newly identified proteins in the nodal, paranodal, and juxtaparanodal regions as potential antigenic targets that could best explain conduction failure and rapid recovery. Cellular and humoral factors, independently or in concert with each other, play a role in the cause of these neuropathies. Although in some of them, there is direct evidence for autoimmune reactivity mediated by specific antibodies or autoreactive T lymphocytes, in others the underlying immune -mediated mechanisms have not been fully elucidated despite good response to immunotherapies22.
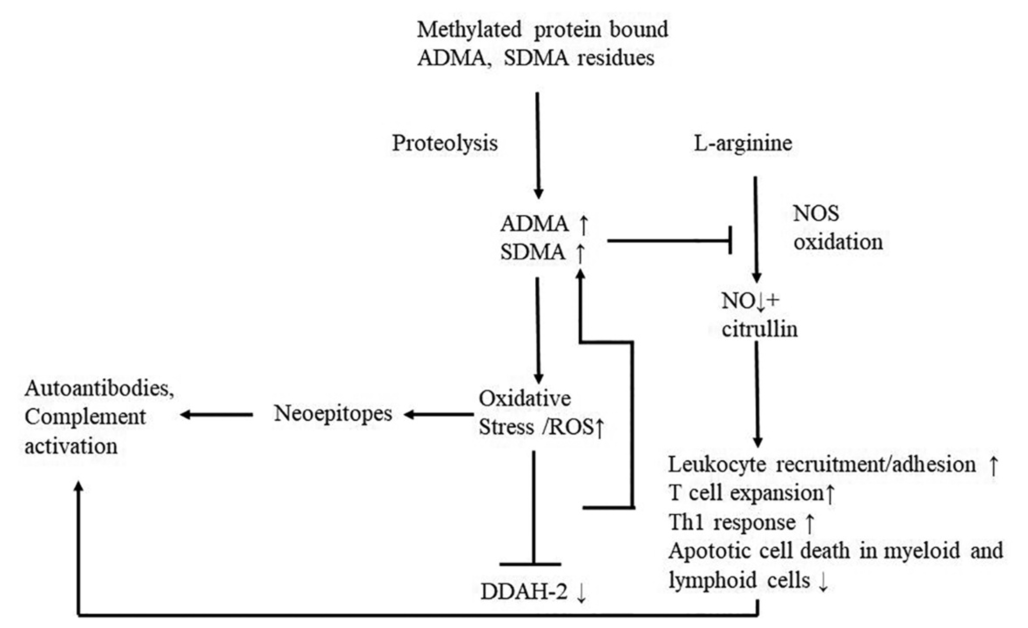
Figure 3. The role of elevated dimethylamine levels in the vicious circle of oxidative stress and inflammation
ADMA: asymmetric dimethylarginine, SDMA: symmetric dimethyl-arginine, DDAH-2: dimethylarginine dimethylaminohydrolase-2, NO: nitric oxide, ROS: reactive oxygen species
Furthermore, recent research suggests that the blockage of NOS aggravates the inflammatory response independently from ROS production. NOS would help limit immune response and contribute to the resolution of inflammation. Various functions of NOS include, among others, the downregulation of leukocyte recruitment23, 24; the blockage of leukocyte adhesion and transendothelial migration25, 26; the inhibition of T cell expansion in lymph nodes, the restriction of Th1 responses, the stimulation of apoptotic cell death in myeloid and lymphoid cells (e.g., effector memory T cells)26, 27.
Elevated ADMA and SDMA levels are tied up with an increased cardiovascular risk and mortality28 and can be found in several renal, pulmonary, cancer, and neurological conditions29. In diabetic neuropathy, the link with increased ADMA levels is more likely to be based on microvascular complications30.
Oxidative stress is a common factor of various polyneuropathies10 and has been shown to lead to detected in eight cases. Increasing evidence suggests that antiganglioside antibodies play a pathogenic role in MMN. Anti-GM1 IgM antibodies isolated from sera of MMN patients have been shown to bind GM1 and active complement in vitro33. IVIG was able to taper the complement deposition in a dose-dependent manner. In peripheral motor nerves, the complement-mediated damage has been affirmed to take place at the nodes of Ranvier. IVIG is effective in treating MMN based on controlled studies34 and significantly reduced ADMA levels in our patients. It comprises IgG antibodies pooled from the serum of thousands of donors. Despite its broad efficacy, its ways of action are still not clear. Cellular and molecular pathways that may be connected to the IVIG-mediated immunosuppression in autoimmune neurological disorders include the blockage of autoantibodies, B cells, complement deposition, T-cell activation factors, Fc-receptors on macrophages, cytokines, chemokines, and adhesion molecules8. In MMN, the blockage of the complement system seems to be the key regulator. IVIG repressed the in vitro complement activation, implying that in vivo the consequential reduction in membrane attack complex (MAC)-mediated injury results the recovery of muscle strength. Even the serum of MMN patients without anti-GM1 antibodies induced IgM binding and complement activation to motor neurites. When pre-treated with IVIG, this process was consider- ably damped35. Complement component C1q and C4 concentrations were markedly reduced in the serum of MMN patients who reached clinical remission on IVIG maintenance therapy33, 36. Thus, the reduction of dimethylamines in our patients might be due to the fact that IVIG limits the positive feedback of inflammation and oxidative stress directly or through effects on neurotransmission. It was also reported that IVIG therapy is associated with scavenging reactive oxygen species in oxida- tive stress35.
STUDY LIMITATIONS AND FUTURE DIRECTIONS
The study population of MMN patients was relatively small due to the rarity of this disease. We cannot completely exclude the effect of chronic diseases (e.g., hypertension, hyperlipidemia; Table 1) and medication on dimethylarginine levels. Nevertheless, patients were successfully treated for their other medical conditions, which should normalize changes in ADMA levels resulting from other diseases to a certain extent. Some drugs, such as statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, lower dimethylarginine levels34. This effect would somewhat compensate for or mask even higher dimethylarginine levels in our patients.
Moreover, after detecting changes in this pilot study, the expansion of the control group, including mimics of MMN (ALS, other pure motor neuropathies) and disorders associated with changes in the dimethylamine pathway, is desirable.
Repeated measurements or serial studies could further explain the mechanisms of changes in ADMA and SDMA levels. These steps could help decide whether dimethylamine levels are helpful parameters to test in connection with the known markers of MMN for the differential diagnosis and progression.
CONCLUSION
MMN is a slowly progressive autoimmune disease, and its cause is unknown. We investigated MMN patients and found elevated serum levels of dimethylarginines. IVIG therapy decreased the serum level of vascular oxidative stress markers ADMA and SDMA and effectively improved the conduction block-related peripheral motor dysfunction right after the treatment. Further experimental models are needed to decide how dimethylarginines are linked to the disease pathomechanism and changed by IVIG treatment.









COMMENTS
0 comments