The eLitMed.hu medical portal uses computer cookies for convenient operation. Detailed information can be found in the Cookie-policy.
Hungarian Immunology - 2007;6(03)
Content
[Adalimumab treatment in inflammatory joint diseases]
[The development of anti-TNF-α agents represents a great advance in the treatment of inflammatory joint diseases. Adalimumab is the first fully human, recombinant IgG1 monoclonal antibody that blocks the interaction of TNF with the p55 and p75 cell surface TNF receptors, thereby neutralising the activity of this cytokine. In well designed, placebocontrolled trials adalimumab significantly reduced symptoms, improved quality of life, and reduced radiologically evident joint damage in patients with rheumatoid athritis, ankylosing spondylitis and psoriatic arhritis. The drug was generally well tolerated, and the follow up studies confirmed, that the incidence of serious adverse events was similar to that generally seen in patients not receiving anti-TNF agents. This review summarises the recent available data related to the efficacy and safety of adalimumab in inflammatory joint diseases.]
[Adalimumab in the treatment of Crohn’s disease]
[Crohn’s disease (CD) is a chronic inflammatory disorder which may involve any part of gastrointestinal tract. The pathogenesis is only partially understood; various environmental and host (e.g. genetic-, epithelial-, immune and non-immune) factors are involved resulting in chronic uncontrolled inflammation, and among pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) seems to play a central role in CD. The last few years have witnessed a significant change in the management of Crohn’s disease. The role of and indications for conventional therapy (aminosalicylates, steroids and immunomodulators) have been reassessed. Over the past decade the increasing knowledge on the pathogenesis of CD led to the development of a number of biological agents targeting specific molecules involved in gut inflammation, first of all TNF-α and its receptors. The aim of this paper is to review the rationale for the use one of the new anti TNF-inhibitors, adalimumab in the treatment of CD.]
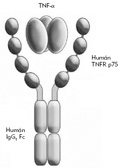
[The importance of etanercept treatment in rheumatology]
[Rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis and psoriatic arthritis are inflammatory rheumatic conditions of unknown origin. Common characteristic features of these disorders include a relatively high prevalence, poorly understood pathogenesis and an unresolved treatment as well as a significant impact on mortality, morbidity and medical expenditures. The recognition of the central role of TNF-α in immune mediated inflammatory conditions, mainly in rheumatoid arthritis has led to the introduction of TNF-α blocking biological therapy into clinical rheumatology revolutionizing the management of these diseases. Etanercept is a human soluble TNF-α receptor attached to human IgG capable of effectively neutralizing TNF-α and lymphotoxin alpha. Since its introduction in 1998 as the first biological agent approved for RA, several clinical trials as well as everyday practice have proven its efficacy and safety. To date approximately 440 thousand patients, mostly with inflammatory rheumatic diseases have been treated with etanercept. In the present paper the pathophysiological role of TNF-α, the results of clinical trials of etanercept and its cost-effectivenes as well as issues regarding the use of etanercept in Hungary are reviewed.]
[Rituximab in rheumatoid arthritis]
[The therapy of rheumatoid arthritis (RA) is not always easy. Classical disease-modifying drugs are ineffective in about 10-15% of the cases. Furthermore, biologic agents, mainly tumor necrosis factor- α (TNF-α) inhibitors, may also be ineffective. Rituximab (RTX) is a B cell-inhibitory monoclonal antibody, which has been registered for the treatment of RA patients refractory to classical immunosuppressive agents including a TNF antagonist. Here we summarize the history of RTX therapy in RA including the presentation of the three major randomized clinical trials. We discuss the efficacy, safety of RTX, the practical points of RTX therapy, as well as some special considerations. The presented data suggest that RTX is a highly effective and safe biological, which can be used upon the inefficacy of any TNF inhibitor. RTX suppresses RA-associated inflammation, symptoms and decreases radiological progression. It may improve the functional capacity and quality of life of RA patients.]
[First experience with rituximab treatment in rheumatoid artritis: a case report of a multiresistant patient]
[INTRODUCTION - Here we describe the case of the first Hungarian rheumatoid arthritis (RA) patient treated with RTX. CASE REPORT - This multiresistant patient had received numerous immunosuppressive drugs and all three anti-TNF agents had been tried. These biologicals had to be stopped due to inefficacy or side effects. RTX treatment resulted in some subjective clinical improvement, as well as a decrease in rheumatoid factor and anti-CCP production. Clinical activity assessed by DAS28 fell after 18 weeks. B cells disappeared from the circulation, however, the percentage of activated T cells increased. We observed initial B cell recovery after 18 weeks. CONCLUSION - Clinical studies suggest that RTX is more effective right after the failure of the first TNF inhibitor. Efficacy of RTX in this patient suggests that this drug may also be effective in a multiresistant patient, who had tried numerous TNF blockers.]
1.
Clinical Neuroscience
[Headache registry in Szeged: Experiences regarding to migraine patients]2.
Clinical Neuroscience
[The new target population of stroke awareness campaign: Kindergarten students ]3.
Clinical Neuroscience
Is there any difference in mortality rates of atrial fibrillation detected before or after ischemic stroke?4.
Clinical Neuroscience
Factors influencing the level of stigma in Parkinson’s disease in western Turkey5.
Clinical Neuroscience
[The effects of demographic and clinical factors on the severity of poststroke aphasia]1.
2.
Clinical Oncology
[Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up]3.
Clinical Oncology
[Pharmacovigilance landscape – Lessons from the past and opportunities for future]4.
5.