The eLitMed.hu medical portal uses computer cookies for convenient operation. Detailed information can be found in the Cookie-policy.
Clinical Oncology - 2019;6(01)
Content
[Practical use of meta-analyses in predicting disease risk, outcome, and therapy response in breast cancer]
[Germinal BRCA status infl uences patient care both in early and advanced/metastatic breast cancer. Ideally, the patient should make the decision on the type of surgery or the avoidance of radiotherapy being aware of the BRCA status; based on the most recent clinical studies, this knowledge may infl uence the type of chemotherapy in the neoadjuvant, adjuvant, or metastatic setting or may raise the use of emerging targeted therapies. DNA-targeting cytostatic agents, mostly platinum agents and PARP inhibitors that act by inducing synthetic lethality, provide specifi c therapies in BRCA-mutant cases. The optimum place and sequence of these specifi c agents in treatment, however, are not known yet. International guidelines promote BRCA testing for the specifi cation of treatment strategy in all HER2-negative advanced/metastatic breast cancer cases (NCCN) or at least in all cases when, based on certain predictors, the presence of mutations is likely (ESMO). Recently, the methods employed for BRCA testing have improved immensely and are widely available through the services of various providers. For the identifi cation of the mutation, sequencing of the whole genes is needed, which can be achieved faster and more cost-effi ciently using next-generation sequencing (NGS) platforms compared to previous methods. It is the responsibility of the physician to consider the possibility of BRCA mutations and to raise the issue of BRCA testing to the patient if the family history, the age, previous malignant disease(s) of the patient, or the cancer features are suggestive of genetic risk.]
[The role of the microbiome in the etiology and treatment of neoplastic diseases]
[Experimental data on the role of the microbiome in the onset and progression of infl ammatory diseases and cancer have been accumulated for years. An important milestone in this respect was the discovery that APC mutant mice in sterile conditions do not develop colon cancer of the FAP type. The direct role of the Enterobacteriaceae and Fusobacteriaceae bacterial families were also shown in the pathomechanism of the same experimental model. The toxic effect of chemotherapy on the gut fl ora has been well documented, but it may very well be that this putative side effect is part of the effi cacy, primarily in the case of adjuvant chemotherapy. The fi rst reproducible methods of microbiome molecular diagnostics are already available today. In addition to standard large clinical studies, we can increasingly rely on evidence of molecular pathomechanism and „real-world” clinical experience in the clinical interpretation of the microbiome. The overview summarizes the results of the fi eld research and its translation possibilities in terms of routine clinical practice.]
[Practical use of meta-analyses in predicting disease risk, outcome, and therapy response in breast cancer]
[Breast cancer is globally the most frequent malignant disease in women with increasing incidence. Meta-analyses using data from a large set of patients combining genetic and standard clinicopathological features provide valuable models in predicting disease risk, outcome and therapy response. With the advent of molecular technologies, the amount of available data generated for each tumor and each patient is growing exponentially. The increased data availability allows the development of new innovative systems enabling to discover more effective prognostic and predictive biomarkers. The goal of this review is to summarize meta-analyses that utilize data from countless patients to provide breast cancer risk prediction (Gail-model, Claus-model, BRCApro, IBIS, BOADICEA), and prediction of prognosis and expected therapy response (PREDICT, Magee). In the last part of the review we introduce online analytical tools (KMplot, ROCplot) developed to examine, rank and validate new prognostic and predictive biomarker candidates.]
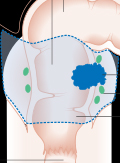
[Molecular subtypes and the evolution of treatment decisions in metastatic colorectal cancer]
[Colorectal cancer (CRC) has clinically-relevant molecular heterogeneity at multiple levels: genomics, epigenomics, transcriptomics and microenvironment features. Genomic events acquired during carcinogenesis remain drivers of cancer progression in the metastatic setting. For example, KRAS and NRAS mutations defi ne a population refractory to EGFR monoclonal antibodies, BRAFV600E mutations associate with poor outcome under standard therapies and response to targeted inhibitors in combinations, while HER2 amplifi cations confer unique sensitivity to double HER2 blockade. Multiple rare gene alterations driving resistance to EGFR monoclonal antibodies have been described with signifi cant overlap in primary and acquired mechanisms, in line with a clonal selection process. In this context, sequential analysis of circulating tumor DNA has the potential to guide drug development in a treatment refractory setting. Rare kinase fusion events and complex alterations in genes involved in DNA damage repair have been described, with emerging evidence for targetability. On the other hand, transcriptomic subtypes and pathway activation signatures have also shown prognostic and potential predictive value in metastatic CRC. These markers refl ect stromal and immune microenvironment interactions with cancer cells. For example, the microsatellite instable (MSI) or POLE ultramutant CRC population is particularly sensitive to immune checkpoint inhibitors, while tumors with a mesenchymal phenotype are characterized by activation of immunosuppressive molecules that mandate stratifi ed development of novel immunotherapy combinations. In this manuscript we review the expanding landscape of targetable oncogenic alterations and signatures in metastatic CRC and discuss the clinical implementation of novel molecular diagnostic tests.]
[P53 – the suppressor]
[Our basic nature requere cells quantity and quality to perform differenciate activity. p53 has the responsibility for quick out those cells who carries molecular failures in DNA avoiding transfer mutations into doughter cells. If the DNA-repair insuffi cient p53s with on apoptosis. Whe p53 is mutated the phenotypes are different in a wide range due to the heterogenity of the DNA damages, and also the expression pattern of a suppressor protein. With the increasing amout the damaged DNA the genomic instability elevates D the risk to development of tumors. It is linict mutated gene could be a promosing tr, 10t for therapy. So far the attempts have little value for the clinic.]
[The role of artifi cial intelligence in precision medicine]
[The essence of practicing medicine has been obtaining as much data about the patient’s health or disease as possible and making decisions based on that. Physicians have had to rely on their experience, judgement, and problem-solving skills while using rudimentary tools and limited resources. With the cultural transformation called digital health, disruptive technologies have started to make advanced methods available not only to medical professionals but also to their patients. These technologies such as genomics, biotechnology, wearable sensors, or artifi cial intelligence (AI) are gradually leading to three major directions. They have been (1) making patients the point-of-care; (2) created a vast amount of data that require advanced analytics; and (3) made the foundation of precision medicine. Instead of developing treatments for populations and making the same medical decisions based on a few similar physical characteristics among patients, medicine has shifted toward prevention, personalization, and precision. In this shift and cultural transformation, AI is the key technology that can bring this opportunity to everyday practice.]
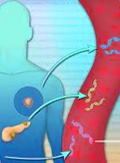
[Liquid biopsy in clinical oncology – fine-tuning precision medicine]
[The classical method of genetically characterising a tumour requires tissue biopsy with which a small sample is removed from the affected organ. This sample represents the tumour in the further analyses. However, the localised nature of sample collection limits representative characterisation. The so-called circulating tumour DNA, isolated from blood plasma after a simple sample collection, potentially enables the oncological analysis of all tumour tissues carrying genetic alterations that can be identifi ed as markers. In order to maximally exploit the potentials of circulating tumour DNA, we must adjust the analytical tools to its specifi c features. The preanalytical handling and storage of the sample signifi cantly infl uences its further usability. In order to be able to detect a potential mutation in a mostly wild-type background, the development of new, specifi c methods is needed, most of which are based on next-generation sequencing techniques. In the past decades, the pronounced decrease in the costs of such techniques led to an accumulation of an immense amount of genetic information on tumorigenesis. Due to the development of sequencing technologies, the turnaround times of tests also decreased enabling their employment in routine care besides research. Starting from our research, this can be realised via three approaches: technological development, the implementation of our already existing diagnostic methods in liquid biopsy, and the construction of well-planned disease-specifi c gene panels. Based on international trends and our experience in serum diagnostics, we are certain that liquid biopsy will become a central pillar of oncological screening and precision oncology in the near future.]
1.
Clinical Neuroscience
[Headache registry in Szeged: Experiences regarding to migraine patients]2.
Clinical Neuroscience
[The new target population of stroke awareness campaign: Kindergarten students ]3.
Clinical Neuroscience
Is there any difference in mortality rates of atrial fibrillation detected before or after ischemic stroke?4.
Clinical Neuroscience
Factors influencing the level of stigma in Parkinson’s disease in western Turkey5.
Clinical Neuroscience
[The effects of demographic and clinical factors on the severity of poststroke aphasia]1.
2.
3.
4.
5.